Aage Niels Bohr
Aage Niels Bohr was born in Denmark’s capital city, Copenhagen, on June 19, 1922.
Aage Niels Bohr was born in Denmark’s capital city, Copenhagen, on June 19, 1922.
In the same year as Aage was born, his father, Niels Bohr, was awarded the Nobel Prize in Physics for his explanation of the structure of atoms and the radiation emitted by them.
Aage’s mother, Margrethe Nørlund, gave birth to six children – all boys; Aage was the fourth. Margrethe was well educated; she assisted Niels Bohr with his paperwork and discussed his scientific research with him in detail.
Aage Bohr’s education was both conventional and, from a scientific point of view, extraordinarily privileged. Like many other students of high school age in Copenhagen, he attended grammar school – the Sortedam Gymnasium. Unlikeother students, he also enjoyed conversations with some of the world’s most outstanding physicists, including his father, of course.
In later life Aage recalled some of the giants of science who had worked in Copenhagen with his father; he met them so regularly that they became his ‘uncles’ – including Uncle Werner Heisenberg (Nobel Prize in Physics 1932) and Uncle Wolfgang Pauli (Nobel Prize in Physics 1945).
Aage Bohr’s Scientific Work
Like his father, Aage Bohr was intrigued by the structure of the atom. The atomic nucleus in particular; that tiny, densely packed, positively charged mass at the heart of every atom interested him intensely.
What was the nucleus really like – were there any structural details, and if so, what were they?
The Nucleus as a Drop of Liquid
One idea, which had been developed most fully by Niels Bohr and John Archibald Wheeler in the late 1930s, was the liquid-drop model. The liquid-drop model pictured the nucleus as a rotating drop of incompressible liquid held together by surface tension.
The drop of liquid could be deformed from its basic spherical shape and a large drop of liquid could fall apart to form two new drops. Similarly a large atomic nucleus, like uranium, could fall apart to form two new atomic nuclei – this is nuclear fission, the energy source behind both the uranium atom bomb and the uranium power plant.
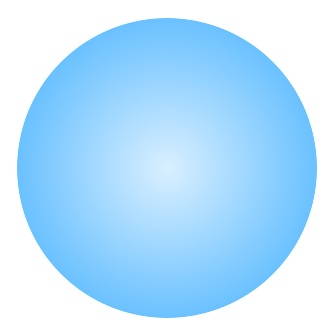
The tiny atomic nucleus was modeled as a drop of liquid held together by surface tension. Just like a liquid, the shape of the drop was spherical, but could be deformed from this shape.
The liquid drop model had its greatest successes in explaining the properties of heavy nuclei, such as uranium.
By 1950, however, the liquid drop model was in danger of being pushed aside by the newer shell model of the nucleus.
The Nucleus with Energy Shells
Much like electrons are said to occupy shells of different energy outside the nucleus, the shell model of the nucleus says protons and neutrons occupy distinct energy shells inside the nucleus.
Shell model of an atomic nucleus, showing different energy levels. Image by Schunck.
By 1950, most physicists had decided the shell model looked more promising than the liquid-drop model.
In particular, the shell model explained why atomic nuclei with so-called magic numbers of protons+neutrons are particularly stable. This is similar to the concept taught in high school chemistry, where atoms with complete electron shells, for example, 2 or 8 electrons in their outermost shells are particularly stable, leading to the unreactive behavior of the noble gases.
In the case of atomic nuclei, the magic numbers of 2, 8, 20, 28, 50, 82 and 126 protons+neutrons result in particularly stable nuclei.
The shell model was particularly good at explaining the properties of lighter nuclei and nuclei with the magic numbers of protons+neutrons, but was less successful with heavy nuclei such as uranium.
 “Today, it is difficult to fully imagine the great impact of the evidence for nuclear shell structure on the physicists brought up with the concepts of the liquid-drop.”
“Today, it is difficult to fully imagine the great impact of the evidence for nuclear shell structure on the physicists brought up with the concepts of the liquid-drop.”
AAGE BOHR, 1975
Nobel Lecture
Unification
In fact, the liquid-drop model and the shell model both had advantages and disadvantages – indicating that neither could be the full story.
In 1949, James Rainwater, a Columbia University physicist, decided to combine the best aspects of the liquid-drop and shell models into a single unified model of the nucleus.
At that time Rainwater shared an office at Columbia with Bohr and explained his ideas to him. Bohr was captivated, seeing the potential of Rainwater’s ideas to explain the behavior and structure of the atomic nucleus.
Bohr returned to Copenhagen, determined to pursue the unified model further. There he worked with Ben Mottelson, who had completed his Ph.D. at Harvard University and who was now in Copenhagen on a Traveling Fellowship from Harvard.
Together, Bohr and Mottelson worked out in intricate detail how a unified model could explain a huge number of experimental observations from different atomic nuclei. In 1953 they published a 173-page report describing their unified model and in 1954 Bohr published The Rotational States of Atomic Nuclei. Crucially, predictions they made about how nuclei would behave were verified in experiments.
One of their key findings was that some of the behavior of nuclei could be explained by nuclei having different amounts of energy resulting from rotation. Furthermore, nuclei do not rotate as rigid objects but, instead, a surface wave travels around the nucleus. They also found that nuclei vibrate, changing their shape around an average value.
At first Bohr had trouble convincing his father that the liquid-drop model should be dropped – after all, Niels Bohr was one of the liquid-drop model’s main architects – but eventually he won his father round.
The unified model – often called the collective model – is sometimes likened to a swarm of bees, where each bee is a neutron or proton and the swarm is the nucleus. The swarm acts as a single entity, even though each bee within it is moving around independently with its own, individual energy. In the Bohr-Mottelson model, the outside of the swarm rotates and wobbles inward and outward.
Each neutron or proton has its own orbital energy within the nucleus. These orbits can sometimes deform the nucleus so that it is no longer truly spherical. For example, the nucleus of heavier atoms can become an oblate spheroid (discus shaped) or prolate spheroid (football shaped).

An oblate and a prolate spheroid. Images by AugPi, modified by this site.
Of course, we need to remember that atomic nuclei have a diameter of between 1.7 x 10−15 m for hydrogen and about 15 x 10−15 m for uranium.
The fact that Bohr and others were able to mathematically model such incredibly small objects, producing fine structural detail, and predicting their behavior in agreement with experimental data is remarkable.
Despite the huge strides taken by the trio of physicists, even today, the structural details of atomic nuclei have still not been fully resolved.

No comments:
Post a Comment
I Am Wating For Your Comments