Alessandro Volta

Alessandro Volta was a physicist, chemist and a pioneer of electrical science. He is most famous for his invention of the electric battery. In brief he:
• Invented the first electric battery – which people then called the “voltaic pile” – in 1800. Using his invention, scientists were able to produce steady flows of electric current for the first time, unleashing a wave of new discoveries and technologies.
• Was the first person to isolate methane.
• Discovered methane mixed with air could be exploded using an electric spark: this is the basis of the internal combustion engine.
• Discovered “contact electricity” resulting from contact between different metals.
• Recognized two types of electric conduction.
• Wrote the first electromotive series. This showed, from highest to lowest, the voltages that different metals can produce in a battery. (We now talk of standard electrode potentials, meaning roughly the same thing.)
• Discovered that electric potential in a capacitor is directly proportional to electric charge.
In recognition of Alessandro Volta’s contributions to science, the unit of electric potential is called the volt.
Early Life and Education
Alessandro Volta was born in Como, Lombardy, Italy, on February 18, 1745. His family was part of the nobility, but not wealthy. Until the age of four, he showed no signs of talking, and his family feared he was not very intelligent or possibly dumb. Fortunately, their fears were misplaced.
When he was seven, his father died leaving unpaid debts. The young Alessandro Volta was educated at home by his uncle until he was twelve years old. He then started studies at a Jesuit boarding school. The Jesuit school charged no fees, but pressurized him to become a priest. His family did not want this, and withdrew him from the school after four years. Volta then studied at the Benzi Seminary until reaching eighteen years of age.
Volta’s family wanted him to become a lawyer. Volta had his own ideas! He was interested in the world around him; he wanted to be a scientist.
Although as a child he had been slow to speak Italian, Volta now seemed to have a special talent for languages. Before he left school, he had learned Latin, French, English and German. His language talents helped him in later life, when he traveled around Europe, discussing his work with scientists in Europe’s centers of science.
Aged 18, Volta was bold enough to begin an exchange of letters about electricity with two leading physicists: Jean-Antoine Nollet in Paris, and Giambatista Beccaria in Turin. Beccaria did not like some of Volta’s ideas and encouraged him to learn more by doing experiments.
When he wrote his first dissertation, Volta addressed it and dedicated it to Beccaria.
 “You must be ready to give up even the most attractive ideas when experiment shows them to be wrong.”
“You must be ready to give up even the most attractive ideas when experiment shows them to be wrong.”
ALESSANDRO VOLTA
Volta’s Career Timeline Before the Battery
Amateur Scientist, Inventor, Teacher and Physics Professor
1765 – Volta had reached 20 years of age. His wealthy friend Giulio Cesare Gattoni had built a physics laboratory in his home. For several years he kindly allowed Volta to do experiments in this laboratory.
1765 – Volta wrote his first scientific paper, which he addressed to Giambatista Beccaria, about static electricity generated by rubbing different substances together – i.e. triboelectricity.
1769 – Volta published a dissertation titled On the Attractive Force of the Electric Fire, and on the Phenomena Dependent On It, which he sent to Beccaria. He discussed his ideas on the causes of electrical attraction and repulsion and compared these with gravity. He set out his position that, like gravity, static electricity involved action at a distance. The main scientists influencing his thinking were Isaac Newton, Roger Boscovich, Benjamin Franklin and Giambatista Beccaria himself.
1771 – Volta read Joseph Priestley’s 1767 review of scientific research on electricity. He learned that some discoveries he had made recently had already been made by others.
1774 – Volta began work overseeing schools in Como. He said that teaching in Como’s classrooms should be modernized. He wanted the children to spend more time learning science and modern languages.
1775 – Volta began teaching experimental physics in Como’s public grammar school, where he worked until 1778.
1775 – Volta wrote a letter to Joseph Priestley. He explained how he had invented a device which was a source of static electricity: the electricity could be transferred to other objects. We call this device the electrophorus. Volta wanted to know if the device was a new invention. Priestly told him Johann Wilcke had invented such a device in 1762, but Volta had invented it independently. Priestley encouraged Volta to keep up his interesting research work.
1776 – Aged 31, Volta was the first person to isolate methane gas. He discovered that a methane-air mixture could be exploded in a closed container with an electric spark. In the future, an electrically started chemical reaction like this would be the basis of the internal combustion engine.
1776 – Volta suggested that the sparking apparatus he used to explode methane could also be used to send an electric signal along a wire from Como to the city of Milan.
 “What is it possible to do well, in physics particularly, if things are not reduced to degrees and measures?”
“What is it possible to do well, in physics particularly, if things are not reduced to degrees and measures?”
ALESSANDRO VOLTA, 1792
1777 – Volta invented a much better eudiometer than any that had gone before. A eudiometer tests how much oxygen is present in air to determine how good for breathing it is. Volta’s eudiometer was superior to others because it used hydrogen as the gas reacting with oxygen, giving a clean, reliable reaction. The reaction was also cleanly started using an electric spark. The eudiometer worked on the basis that the decrease in volume of hydrogen after sparking was proportional to the amount of oxygen present in air.
1777 – Volta set out on a scientific journey to Switzerland and France. He met other scientists and showed them his innovations in electrical equipment. He also traveled so that his name would become better known outside Italy.
1778 – Volta was appointed to the Chair of Experimental Physics at the University of Pavia, about 55 miles (85 km) from Como, a position he would hold for over 40 years.
1778 – Volta discovered that the electrical potential (we now often call this thevoltage) in a capacitor is directly proportional to electrical charge.
1781 to 1782 – Volta traveled around most of Europe’s major scientific centers, including the French Academy in Paris, demonstrating his electrical equipment and inventions to eminent people such as Antoine Lavoisier and Benjamin Franklin. Volta was beginning to become well-known outside Italy.
1782 – Volta wrote about the condenser he had constructed (today we would call it a capacitor) to collect and store electric charge, and how he had used it to study a variety of electrical phenomena.
1788 – Volta built increasingly sensitive electroscopes to detect and measure the effects of electric charge.
1790 – Volta carried out experiments on the behavior of gases. He found an accurate value for air’s increasing volume with rising temperature.
1791 – Recognizing that he had become one of Europe’s foremost electrical scientists, Volta was elected to be a Fellow of the Royal Society of London.
1794 – At the age of 50, Volta was awarded the Royal Society’s top prize – the Copley Medal – for his contributions to scientific understanding of electricity.
Invention of the Electric Battery
A Feud over Frogs’ Legs led to the Battery
Volta did not set out to invent the battery. His experiments in this area were actually performed to show the claims of another scientist were wrong. That scientist was another Italian, Luigi Galvani.
Jumping Frogs’ Legs
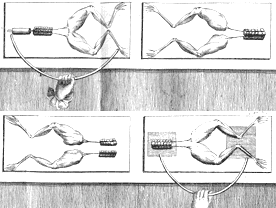
Galvani discovered that contact between frog leg nerves and different metals caused the legs to move. We now understand that he had created an electric cell. The frog legs acted as the electrolyte and also moved when stimulated by the flow of electricity.
This was an amazing discovery: animal movement was based on electricity in some way.
In 1817, this led to Mary Shelley writing Frankenstein. In this novel, a creature made from a monstrous mixture of body parts from dead people is brought to life by Doctor Frankenstein using electricity from a lightning storm.
In 1791, Galvani announced his discovery of animal electricity. He believed that animals generated electricity in their bodies and that a fluid within animals’ nerves carried electricity to muscles, causing movement. He believed that electricity from an outside source released a flow of electrical fluid from the nerves, causing the muscles to jump.
He also believed that animals such as electric eels could build up extra amounts of this fluid and use it to deliver electric shocks.
Galvani concluded that animal electricity was similar to static electricity, but it was different and was a unique property of living things.
Enter Volta
Volta studied Galvani’s phenomenon.
In 1792, Volta said that the “animal” part of Galvani’s animal electricity was not needed. Animals merely responded to normal electricity. There was no difference between animal electricity and electricity.
Volta performed various experiments on frogs’ legs. He found the key to getting them to move was contact with two different metals. Contact with pieces of the same metal did nothing.
Then, moving away from frogs’ legs, in 1794, Volta did experiments to measure the electrical effect of bringing different pairs of metals into contact. He listed the metals in order of what he called their electromotive force.
Volta’s List Of Conductors, Highest Electromotive Force First
Zinc
Lead
Tin
Iron
Copper
Silver
Gold
Graphite
Manganese Ore
Lead
Tin
Iron
Copper
Silver
Gold
Graphite
Manganese Ore
This was the first time anyone had listed electrode potentials. It was the first electrochemical series.
In modern language, we would say that the farther apart the substances on this list are, the greater the voltage they will produce when brought into contact or used as the electrodes in electric cells and batteries. For example, a zinc-graphite cell will produce a greater voltage than a zinc-lead cell.
By 1797, Volta had completely proved his “contact theory” of electricity.
He now knew that the key to producing what today we call a voltage was two metals connected by by something moist, like frogs’ legs. The moist connection between the metals did NOT have to be an animal. Connecting the metals by placing them in a cup of dilute acid was a very effective way of producing electricity.
He formally split electrical conductors into those of the first kind: these were metals, graphite and pure charcoal; and the second kind: these were substances we would now call electrolytes, such as salt water or dilute acids. An electric current would result when a circuit was built using two conductors of the first kind combined with one of the second kind.
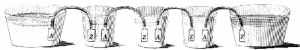
An illustration from Volta’s 1800 paper. Pieces of silver (A) and zinc (Z) connected by metal strips and sitting in cups of dilute acid will produce electricity. This could be tested by putting a finger in each of the end cups. You would get an electric shock. Unlike Galvani’s version, no animals need be hurt in this production, except for the human tester who gets a mild electric shock.
Alternatively, connecting the metals with paper soaked in dilute acid or salt water also worked.
Volta said that in Galvani’s work, the frogs’ legs had served two functions:
- They conducted electricity as conductors of the second kind.
- They acted as a very sensitive electroscope. (An electroscope is a device used to detect electricity.)
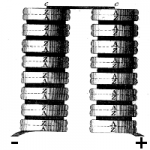
Diagram from Volta’s 1800 paper. The pile is made using discs of silver (A) and zinc (Z) linked in series with card soaked in salt water. The positive and negative polarities of this battery are as shown. Adding more pairs of discs increases the voltage of the battery.
Volta found that by connecting up more and more pairs of metals connected with moist card, he could produce ever higher voltages, leading to significant electrical currents.
And so the electrical battery was born.
Volta used alternating zinc and silver discs linked by card or cloth soaked in salt water.
In 1800, Volta described his results in a letter to Joseph Banks, at the Royal Society in London.
Banks showed the letter to other scientists, and arranged for Volta’s description of his discovery to be read out at a meeting of the Society and published.
 “I continue coupling a plate of silver with one of zinc, and always in the same order… and place between each of these couples a moistened disk. I continue to form a column. If the column contains about twenty of these couples of metal, it will be capable of giving to the fingers several small shocks.”
“I continue coupling a plate of silver with one of zinc, and always in the same order… and place between each of these couples a moistened disk. I continue to form a column. If the column contains about twenty of these couples of metal, it will be capable of giving to the fingers several small shocks.”
ALESSANDRO VOLTA, 1800
Volta’s Battery Unleashed a Wave of New Scientific Discoveries
The battery that Volta had invented gave chemists a very powerful new method to study substances.
The beauty of Volta’s device was that almost anyone could make one – silver and copper coins were available to many people, as were other metals such as iron, tin and zinc.
Within weeks of Volta’s invention of the battery, William Nicholson and Anthony Carlisle built and used a battery to decompose water into hydrogen and oxygen.
Within just six years, Humphry Davy had built a powerful battery. With it, he isolated new chemical elements, and deduced that chemical bonds were electrical in nature.
Volta demonstrates his battery to Napoleon Bonoparte in 1801. Napoleon was very impressed by Volta’s work, giving him the aristocratic title of Count.
Davy’s discoveries of the new elements barium, calcium, lithium, magnesium, potassium, sodium, and strontium, were all made possible by Volta’s invention of the battery.
By 1820, courtesy of Volta’s batteries, Hans Christian Oersted was investigating the relationship between electricity and magnetism.
By 1821, Michael Faraday had produced an electric motor.
Volta’s battery produced a steady source of electric current for the first time ever. All electrical devices depend on electric current. Without Volta’s invention, there could be no modern technology. Volta’s battery was an absolutely crucial invention in the development of our technology based civilization.
The End
In 1819, at the age of 74, Volta decided it was time to hang up his capacitors, his voltaic piles, his electrophorus, and his administrative work at the university. He retired to a country house close to his home town of Como, where he could spend more time with his wife, Maria Teresa. They had three sons, Zanino, Faminio and Luigi.
Volta lived in Como until his death, aged 82, on March 5, 1827.
In 1881, scientists decided that the unit of electric potential would be called thevolt to recognize Volta’s great contributions to electrical science.

No comments:
Post a Comment
I Am Wating For Your Comments