John Dalton

Lived 1766 – 1844.
John Dalton’s Atomic Theory laid the foundations of modern chemistry.
John Dalton’s Early Life and Education
John Dalton was born on September 6, 1766, in Eaglesfield, England.
His father was a weaver, who owned a house and a small amount of land. Both of his parents were Quakers.
Although Quakers were Christians, they were seen as dissenters by the established Church of England. As a result of this, John Dalton’s higher educational opportunities were restricted to dissenting places of education.
John Dalton was an intelligent child, who took an interest in the world around him and tried to learn as much as he could about everything.
He attended his village school until he was 11, and then began helping as a teacher.
At age 15, he started helping his older brother John to run a Quaker boarding-school in the town of Kendal, 40 miles from his home. All the while, he continued teaching himself science, mathematics, Latin, Greek and French. By the time he was 19, he had become the school’s principal, continuing in this role until he was 26 years old.
It seems that the school’s students liked Dalton teaching them, one of them recalling:
“The boys (were) all glad to be taught by John Dalton, because he had a gentler disposition; and besides his mind was so occupied with mathematics, that their faults escaped his notice.”
Becoming a Scientist
In the first half of 1793, aged 26, Dalton took the position of teacher of mathematics and natural philosophy at Manchester’s New College, a dissenting college.
In 1794, he wrote his first scientific paper which he called: Extraordinary Facts Relating to the Vision of Colours.
This was the first ever paper to discuss color blindness. Dalton had realized the condition was hereditary, because he and other members of his family had it.
Ultimately, Dalton’s theory for color blindness was wrong, but as he was the first person ever to research it, the condition became known as Daltonism.
Further research papers followed, in the physical sciences: heat conduction, gas expansion by heat, the properties of light, the aurora borealis, and meteorology.
In 1800, Dalton began earning a living as a private tutor in science and mathematics. He resigned from New College, which was in financial difficulty.
Atomic Theory
The Behavior of Gases
In 1801, Dalton gave a series of lectures in Manchester whose contents were published in 1802. In these lectures he presented research he had been carrying out into gases and liquids. This research was groundbreaking, offering great new insights into the nature of gases.
Firstly, Dalton stated correctly that he had no doubt that all gases could be liquified provided their temperature was sufficiently low and pressure sufficiently high.
He then stated that when its volume is held constant in a container, the pressure of a gas varies in direct proportion to its temperature.
This was the first public statement of what eventually became known as Gay-Lussac’s Law, named after Joseph Gay-Lussac who published it in 1809.
In 1803, Dalton published his Law of Partial Pressures, still used by every university chemistry student, which states that in a mixture of non-reacting gases, the total gas pressure is equal to the sum of the partial pressures of the individual gases.
By now, Dalton’s work had distinguished him as a scientist of the first rank, and he was invited to give lectures to the Royal Institution in London.
Dalton and Atoms
His study of gases led Dalton to wonder about what these invisible substances were actually made of.
The idea of atoms had first been proposed more than 2000 years earlier by Democritus in Ancient Greece. Democritus believed that everything was made of tiny particles called atoms and that these atoms could not be split into smaller particles. Was Democritus right? Nobody knew!
Dalton was now going to solve this 2000 year-old mystery.
He carried out countless chemical reactions, and in 1808 published what we now call Dalton’s Law in his book A New System of Chemical Philosophy:
If two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers.
For example, Dalton found that 12 grams of carbon could react with 16 grams of oxygen to form the compound we now call carbon monoxide or with 32 grams of oxygen to form carbon dioxide. The ratio of oxygen masses of 32:16, which simplifies to 2:1 intrigued Dalton. Analyzing all of the data he had collected, Dalton stated his belief that matter exists as atoms. He went further than Democritus, by stating that atoms of different elements have different masses. He also published diagrams showing, for example:
1. How atoms combine to form molecules
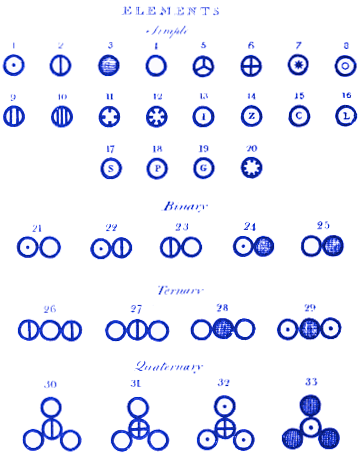
Here, at the top of his diagram, Dalton assigns atom 1 to be hydrogen, 2 nitrogen, 3 carbon, 4 oxygen, 5 phosphorus, etc.
He then shows how molecules might look when the atoms combine to form compounds. For example, molecule 21 is water (OH), 22 is ammonia (NH) and 23 is nitrogen oxide (NO). Of these molecules, the modern reader will notice that Dalton got molecules 21 and 22 wrong.
This was initially less important than the fact that Dalton’s system of atoms and molecules is almost identical to how we might represent them today. For example, Dalton’s molecule 28 is carbon dioxide. Today, we would still draw carbon dioxide in this way.
Amedeo Avogadro soon published work that built on Dalton’s and corrected some of Dalton’s errors – such as finding that water should be written H20 – but unfortunately Avogadro’s work was ignored for many years, partly because it disagreed with Dalton’s.
2. How molecules of water might look in ice
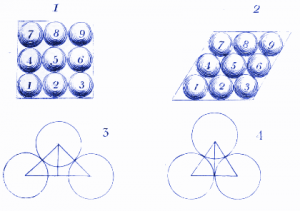
Here Dalton shows how water molecules might arrange themselves when they are frozen in ice. We use similar diagrams today to show how atoms and molecules arrange themselves in crystals.
Dalton’s Atomic Theory states that:
1. The elements are made of atoms, which are tiny particles, too small to see.
2. All atoms of a particular element are identical.
3. Atoms of different elements have different properties: their masses are different, and their chemical reactions are different.
4. Atoms cannot be created, destroyed or split.
5. In a chemical reaction, atoms link to one another, or separate from one another.
6. Atoms combine in simple whole-number ratios to form compounds.
Although we have learned that atoms of the same element can have different masses (isotopes), and can be split in nuclear reactions, most of Dalton’s Atomic Theory holds good today, over 200 years after John Dalton described it. It is the foundation on which modern chemistry has been built.
 “Mr. Dalton’s permanent reputation will rest upon his having discovered a simple principle, universally applicable to the facts of chemistry – in fixing the proportions in which bodies combine, and thus laying the foundation for future labors… his merits in this respect resemble those of Kepler in astronomy.”
“Mr. Dalton’s permanent reputation will rest upon his having discovered a simple principle, universally applicable to the facts of chemistry – in fixing the proportions in which bodies combine, and thus laying the foundation for future labors… his merits in this respect resemble those of Kepler in astronomy.”
HUMPHRY DAVY, 1778 TO 1829
Honors
Dalton did not marry and had no children. He remained a faithful Quaker all of his life, living modestly.
In 1810, he declined an invitation to become a member of the Royal Society. In 1822, he was elected without his knowledge. In 1826, he was awarded the Society’s Royal Medal for his Atomic Theory.
In 1833, the French Academy of Sciences elected him as one of its eight foreign members. In 1834, the American Academy of Arts and Sciences elected him as a foreign member.
The End
When he was 71 years old, Dalton had a small stroke – or paralysis as it was known then. A year later, a more significant stroke left him unable to speak as clearly as he once could. In 1844, when he was 77, another stroke hit him. He died aged 77 on July 27, 1844.
His scientific reputation was so great that when his body was placed in Manchester Town Hall it was visited by more than 40,000 people paying their respects. John Dalton was buried in Manchester in Ardwick cemetery.

No comments:
Post a Comment
I Am Wating For Your Comments